Oracle Product Lifecycle Management (PLM)
Is your product lifecycle management (PLM) software helping you rapidly design and launch new products? Oracle Fusion Cloud PLM is a collaborative platform that helps you get your best ideas to market faster by standardizing and structuring the data and processes that go into innovating, developing, and commercializing products and services.
Create SEO-friendly product descriptions in just a few clicks with embedded generative AI (1:00)
Discover Oracle Product Lifecycle Management.
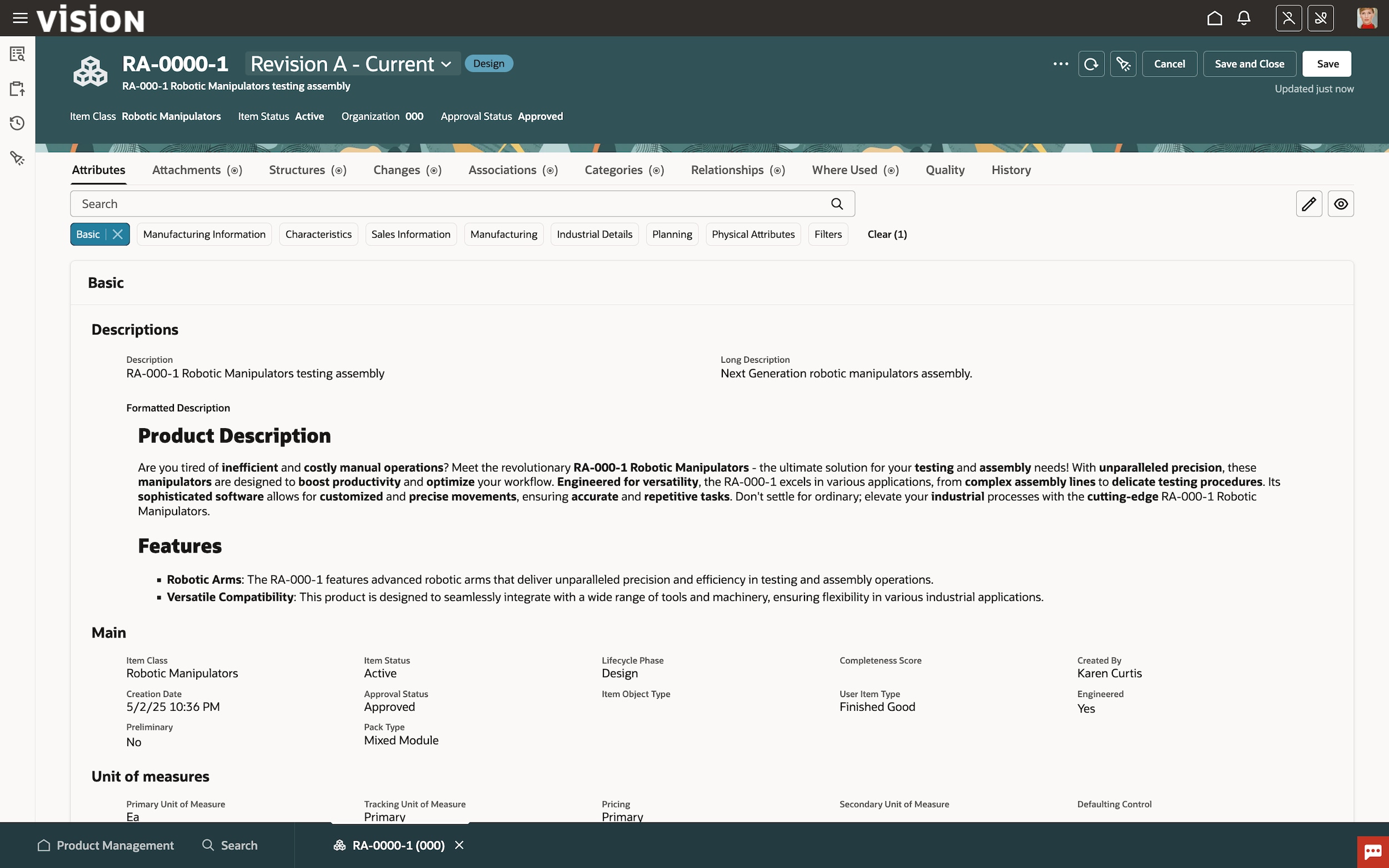
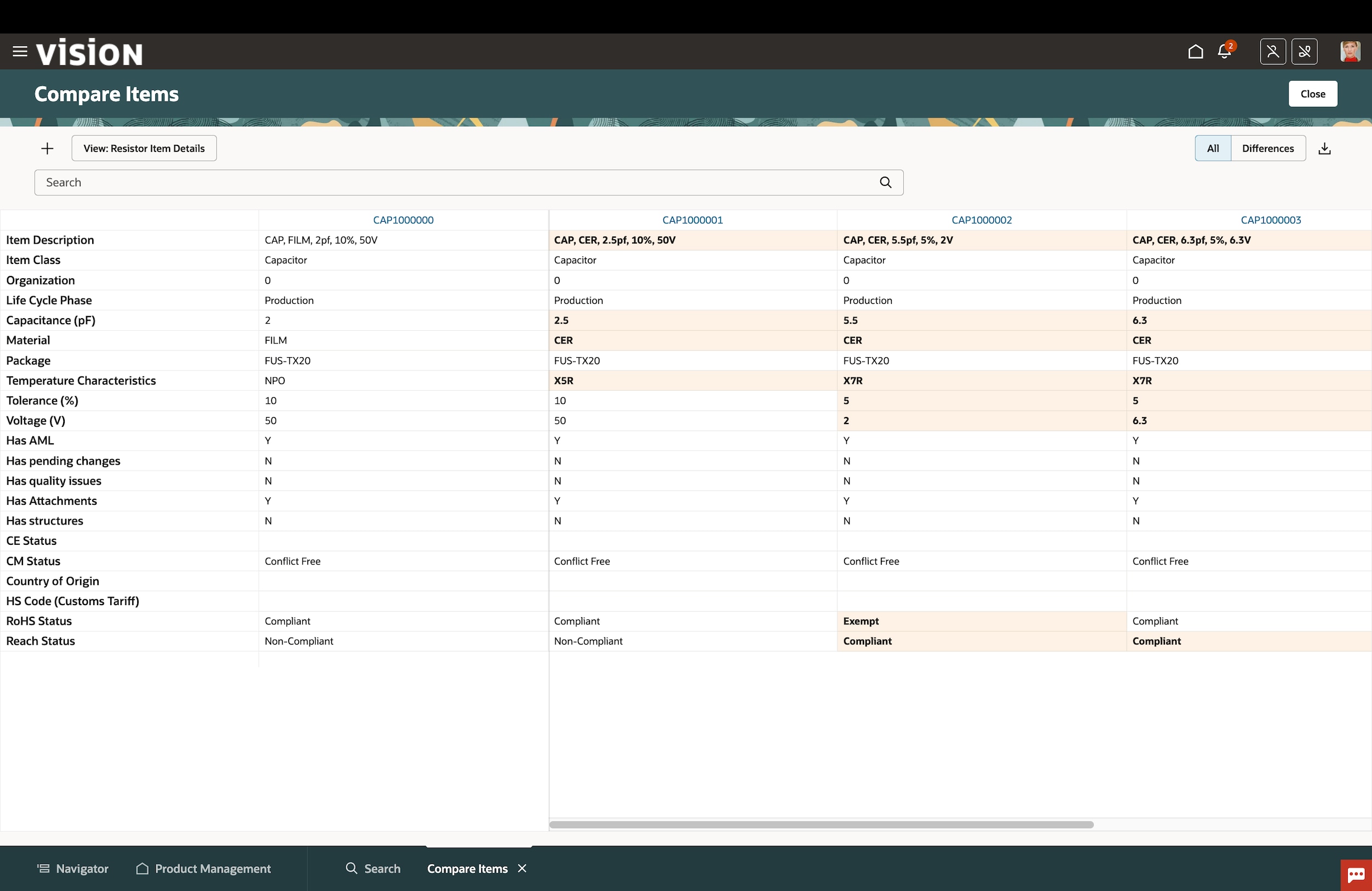
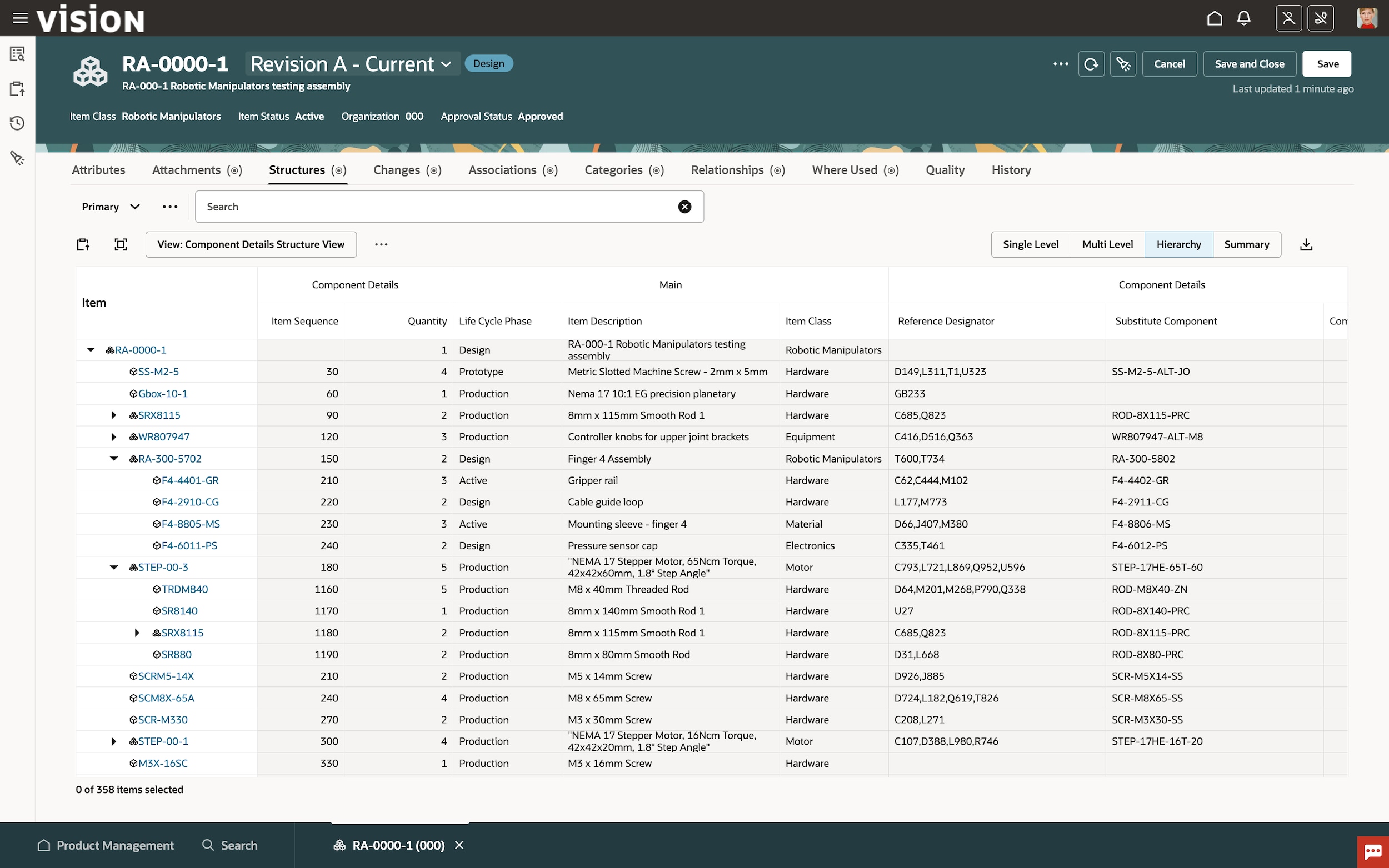
Oracle Fusion Cloud Product Lifecycle Management (PLM)
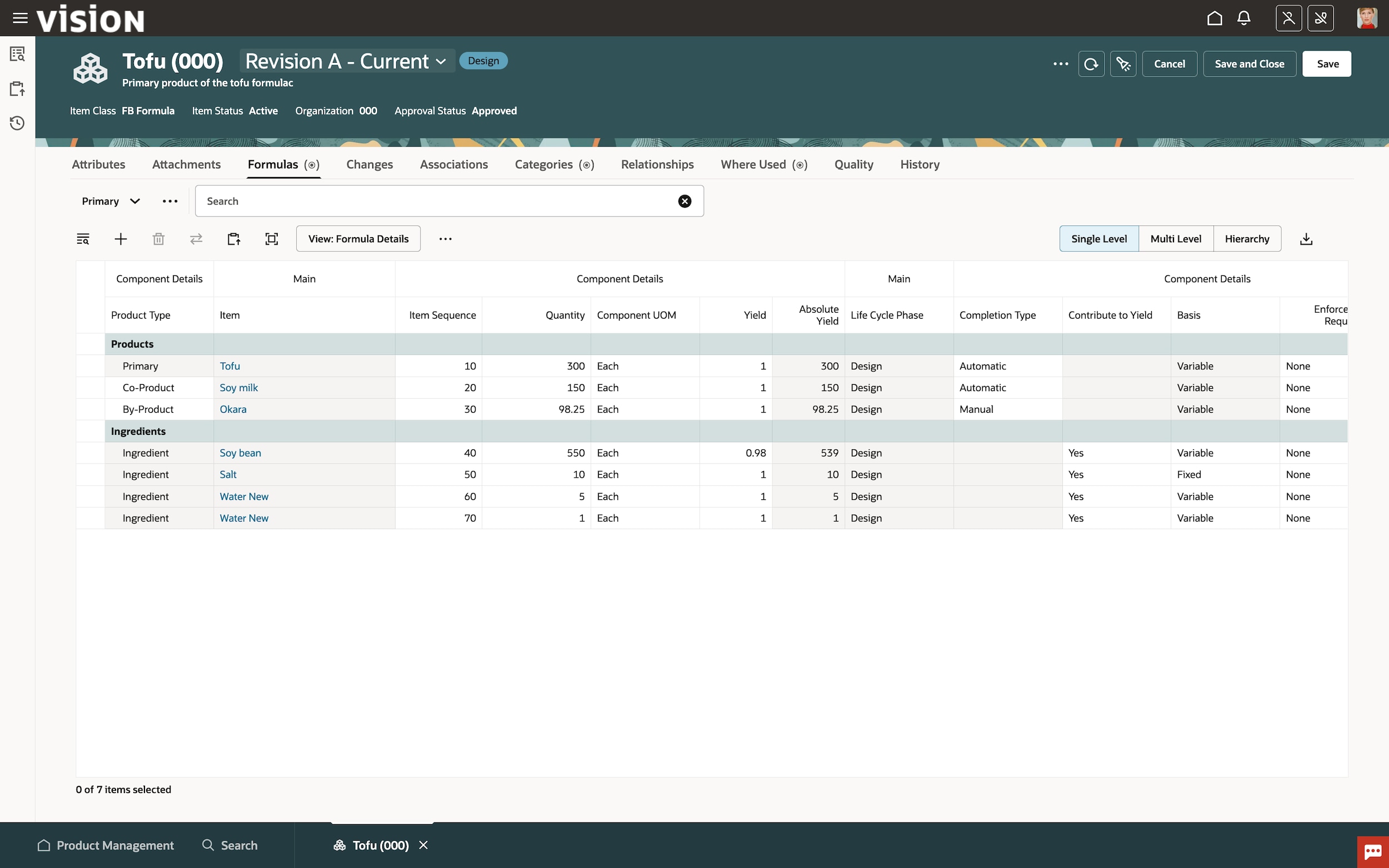
Learn how Oracle Cloud PLM accelerates innovation and new product introductions by efficiently managing items, parts, products, documents, requirements, engineering change orders, and quality workflows across globalized supply chains while seamlessly integrating to computer-aided design (CAD) systems.
Innovation management
Profitable innovations, faster
Drive faster, smarter innovation and ensure sustainable growth. Oracle Cloud PLM helps you maintain a profitable innovation pipeline fueled by a steady stream of the highest-value, on-target, and relevant ideas.
Ideate everywhere
Capture ideas from any source for new products, services, markets, or customer experiences. Evaluate each proposal across a 360-degree perspective of resource needs, assessed value, cost, and constraints.
Manage requirements and concepts
Document, prioritize, and agree on requirements leveraged in developing innovation concepts. Reuse existing items, trace requirements through design, and validate that each has been met to reduce new product introduction risks.
Build agility into innovation portfolios
Balance core, adjacent, and transformational innovation initiatives while aligning your resources, risk mitigation, and budgets. Use best-practice analysis to select an innovation portfolio that achieves your strategic and profit objectives.
Product development
Rapidly develop and launch products
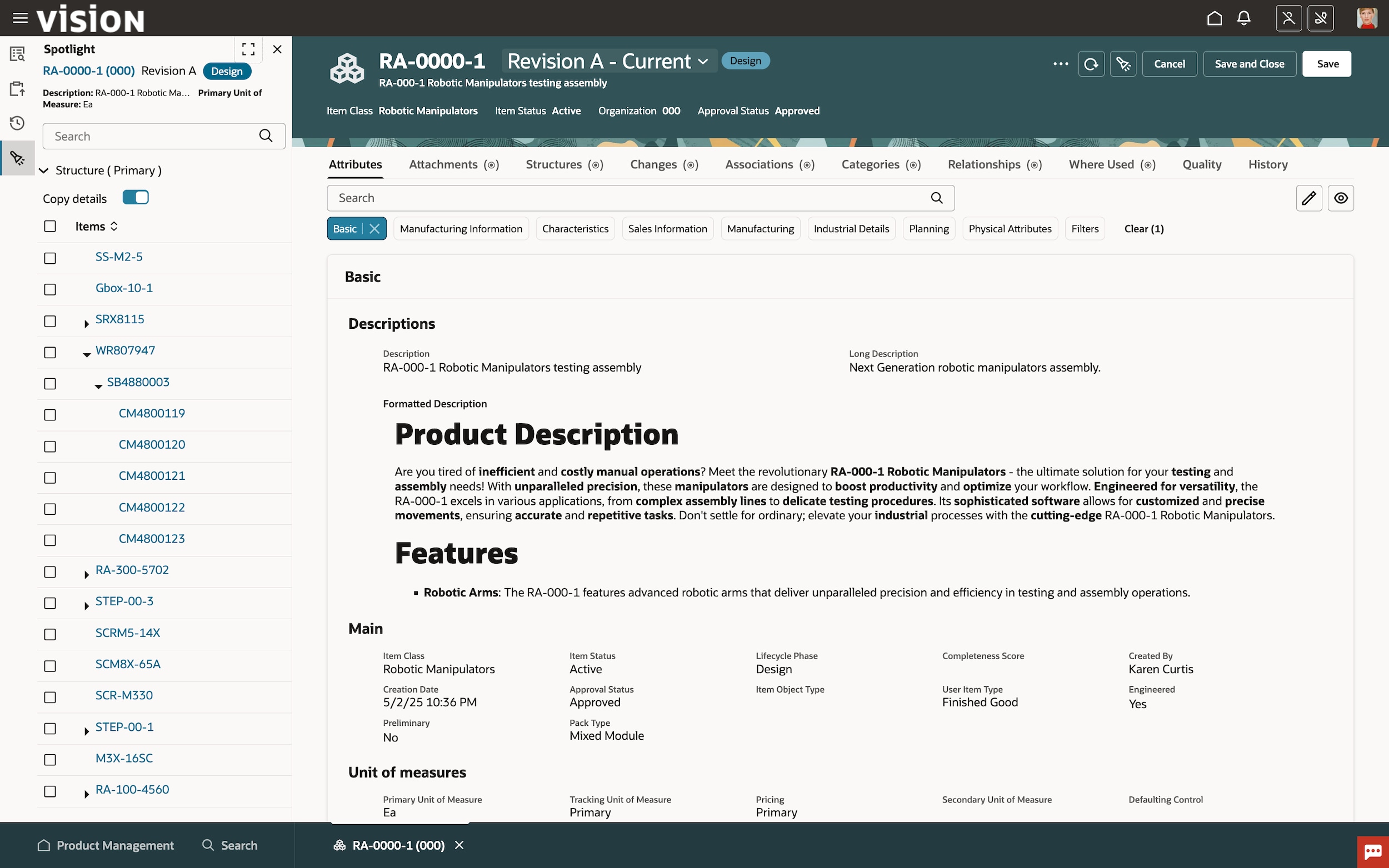
Gain faster, more efficient product development and launch processes by bridging traditional, disconnected engineering and manufacturing functions through streamlined management of an integrated enterprise product record.
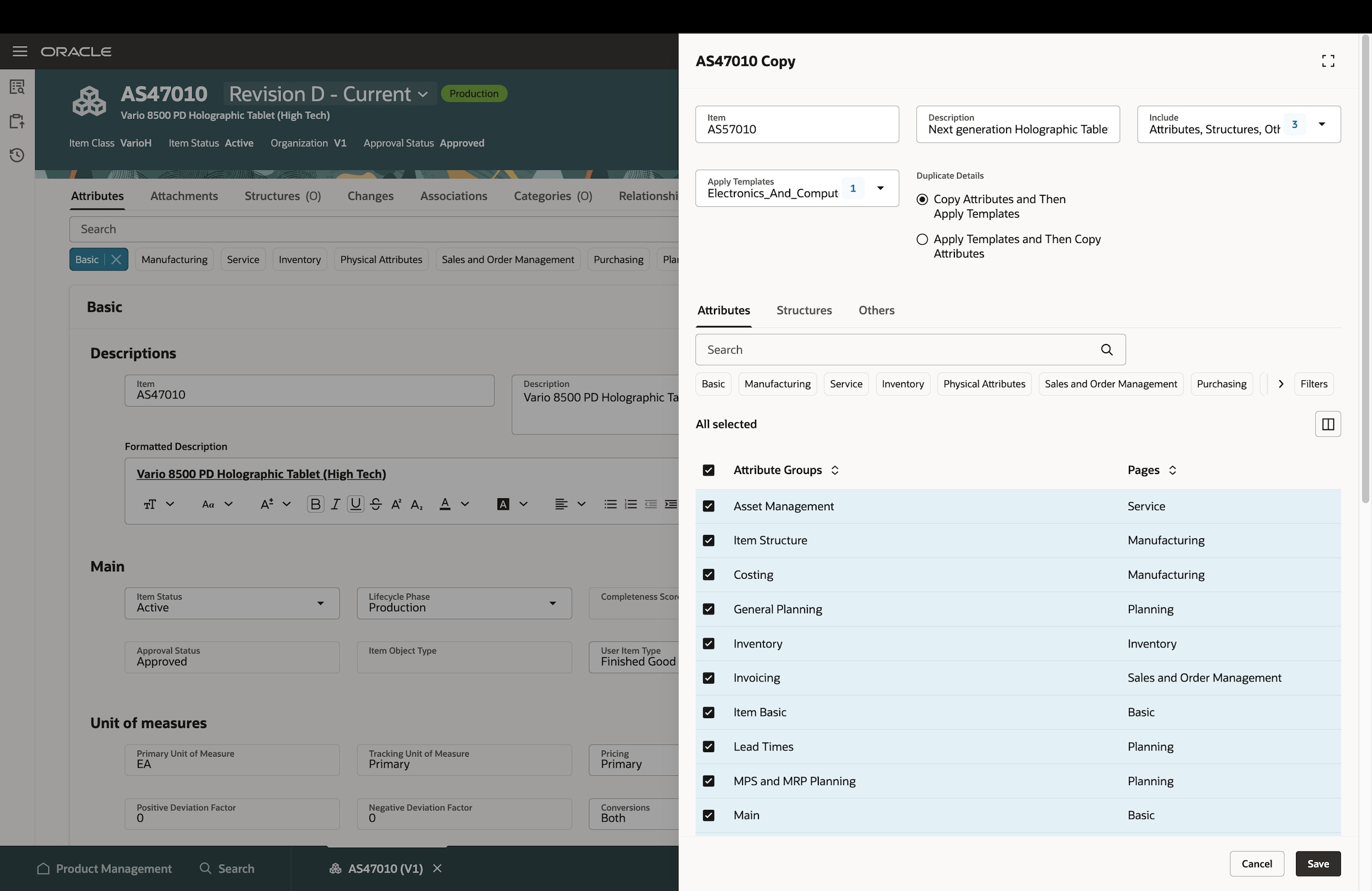
Minimize design cost
Gain complete visibility into your product(s) evolution by monitoring product iterations. Get feedback sooner and avoid costly rework with more effective collaboration internally and with your design partners.
Reduce supply risks
Reduce supply risk and track part preferences for manufacturing throughout each stage of design by maintaining a single view of qualified parts and suppliers across the entire product structure.
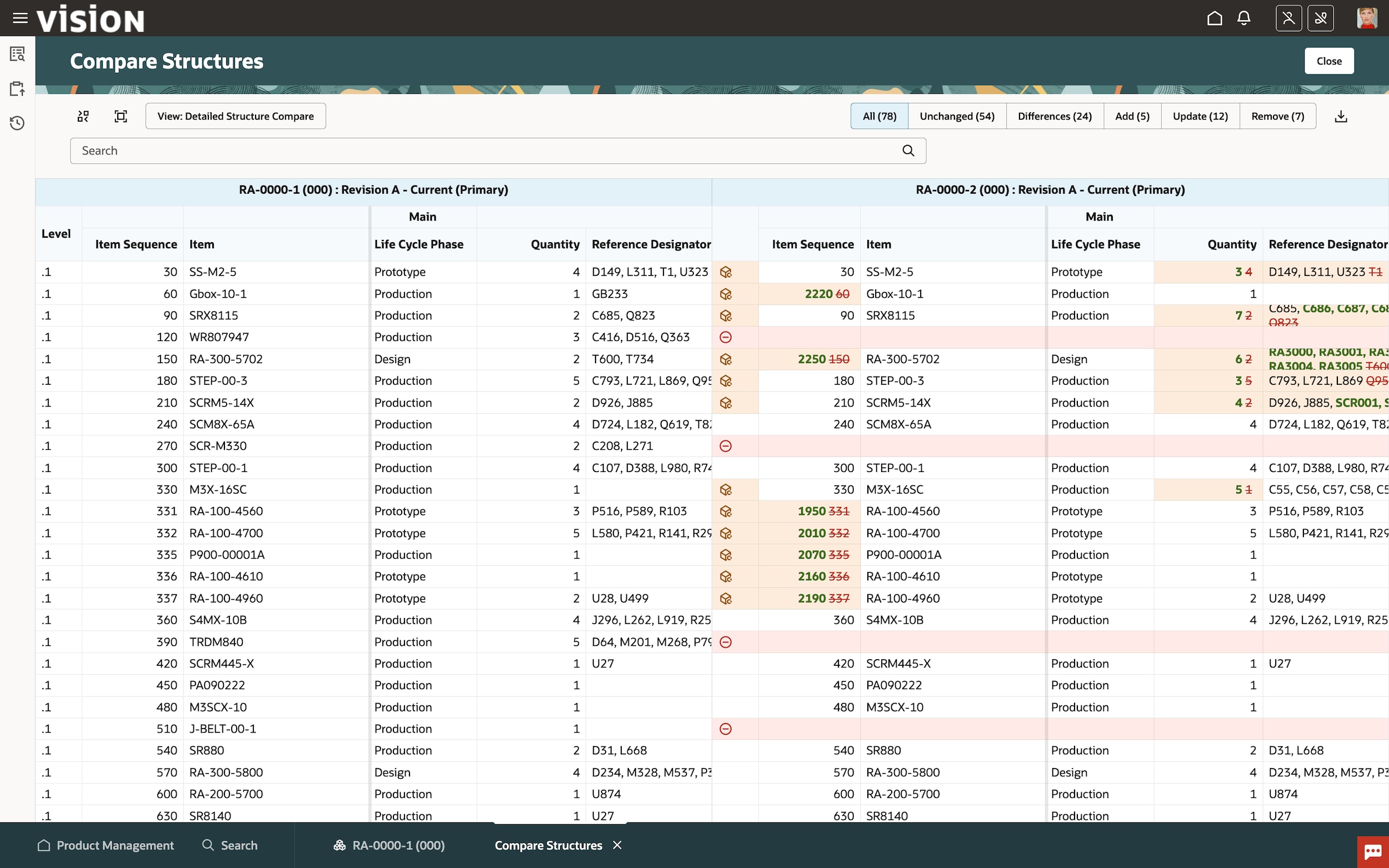
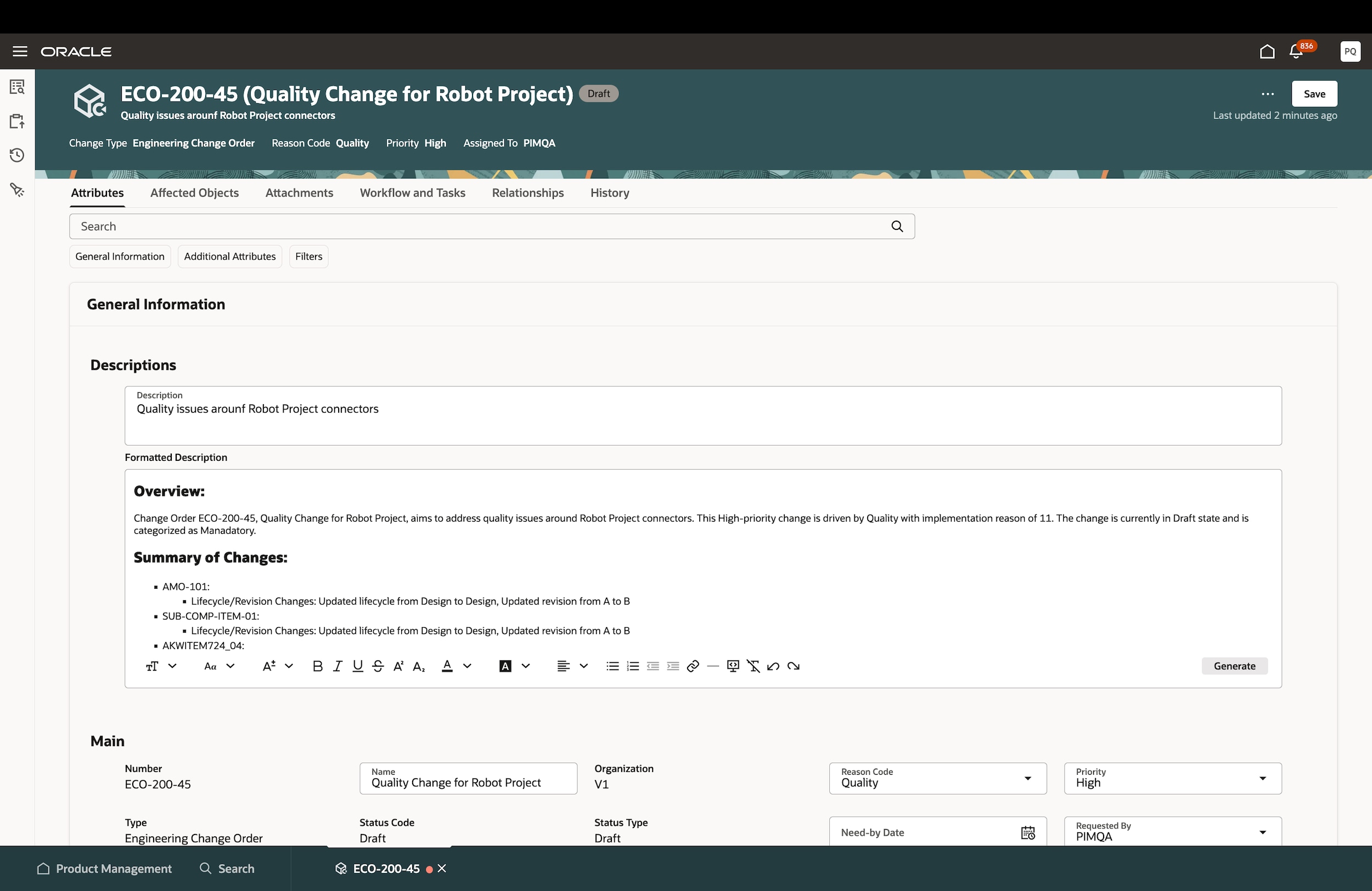
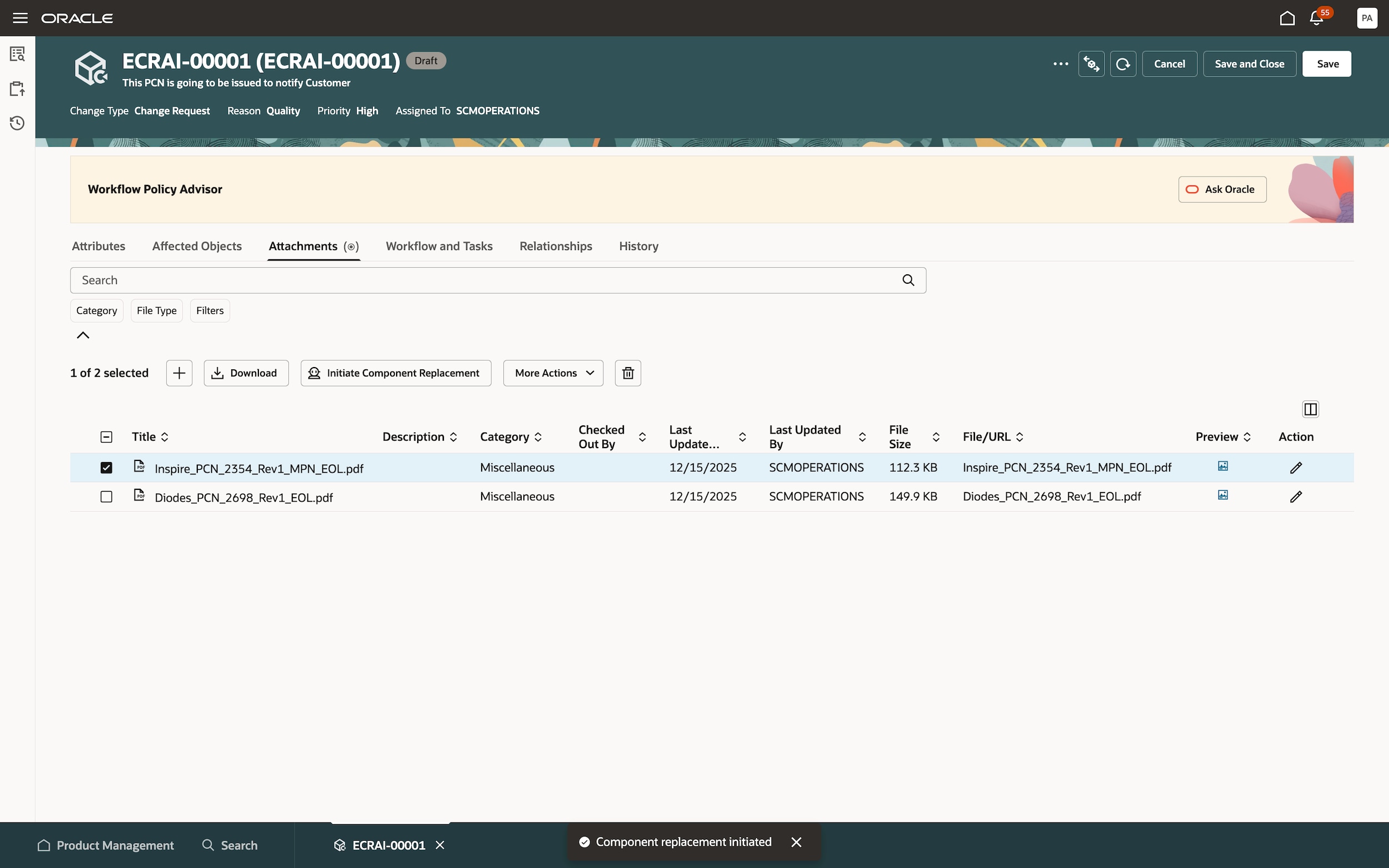
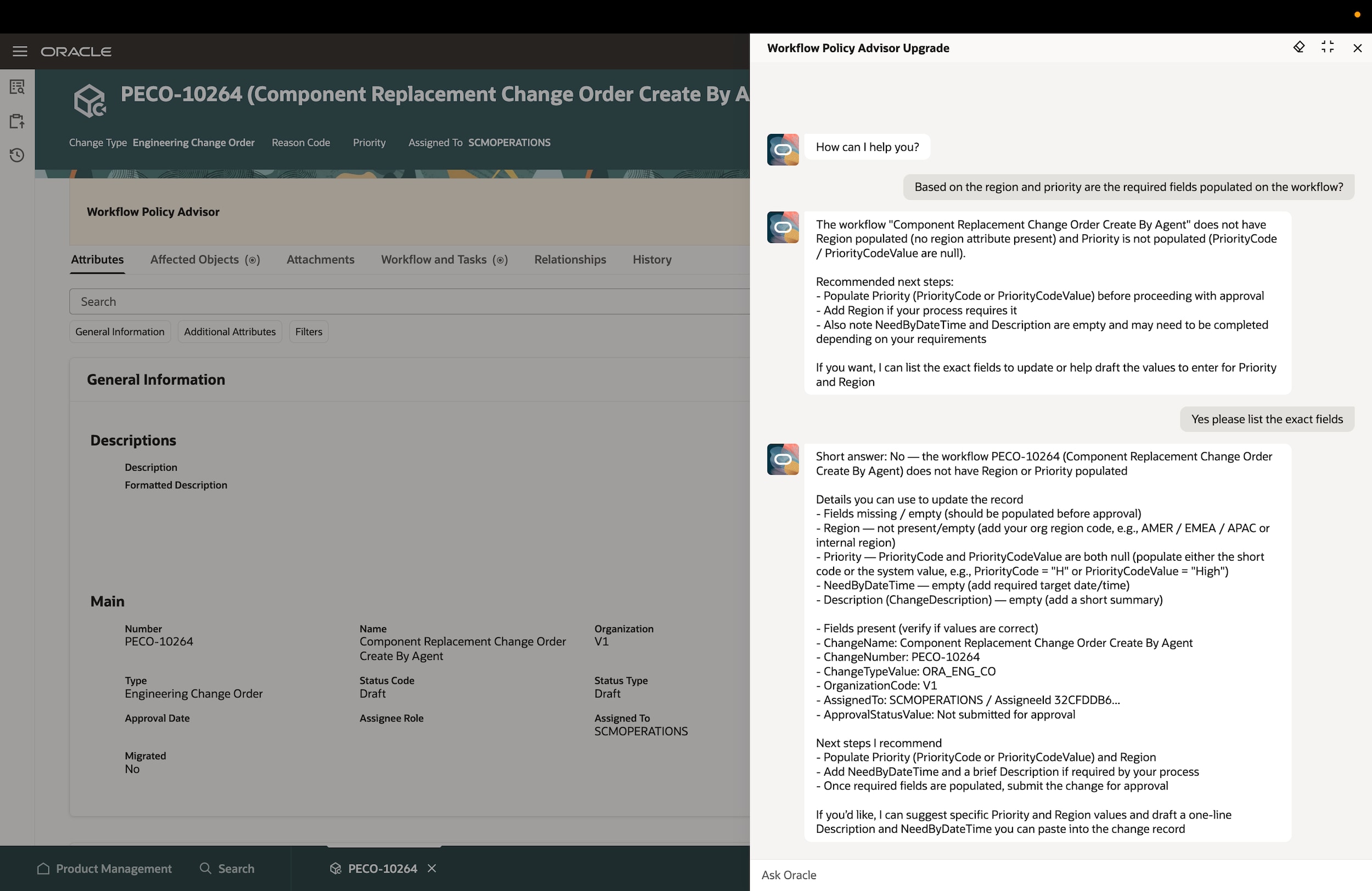
Accelerate change management
Effectively manage and model change iterations with a collaborative change management process. Eliminate rework and compress cycle times by configuring defined workflows with the flexibility to make changes if needed.
Enforce product compliance
Enforce product compliance and proactively track changes throughout your product’s lifecycle so you can quickly adjust to changing global standards, efficiently manage engineering documentation, and design compliant-finished products.
Quality management
Closed-loop quality
Improve the productivity, quality, and profitability of your processes and products. Drive closed-loop quality processes through design, procurement, inventory, manufacturing, and field service to ensure rapid detection and resolution of quality events.
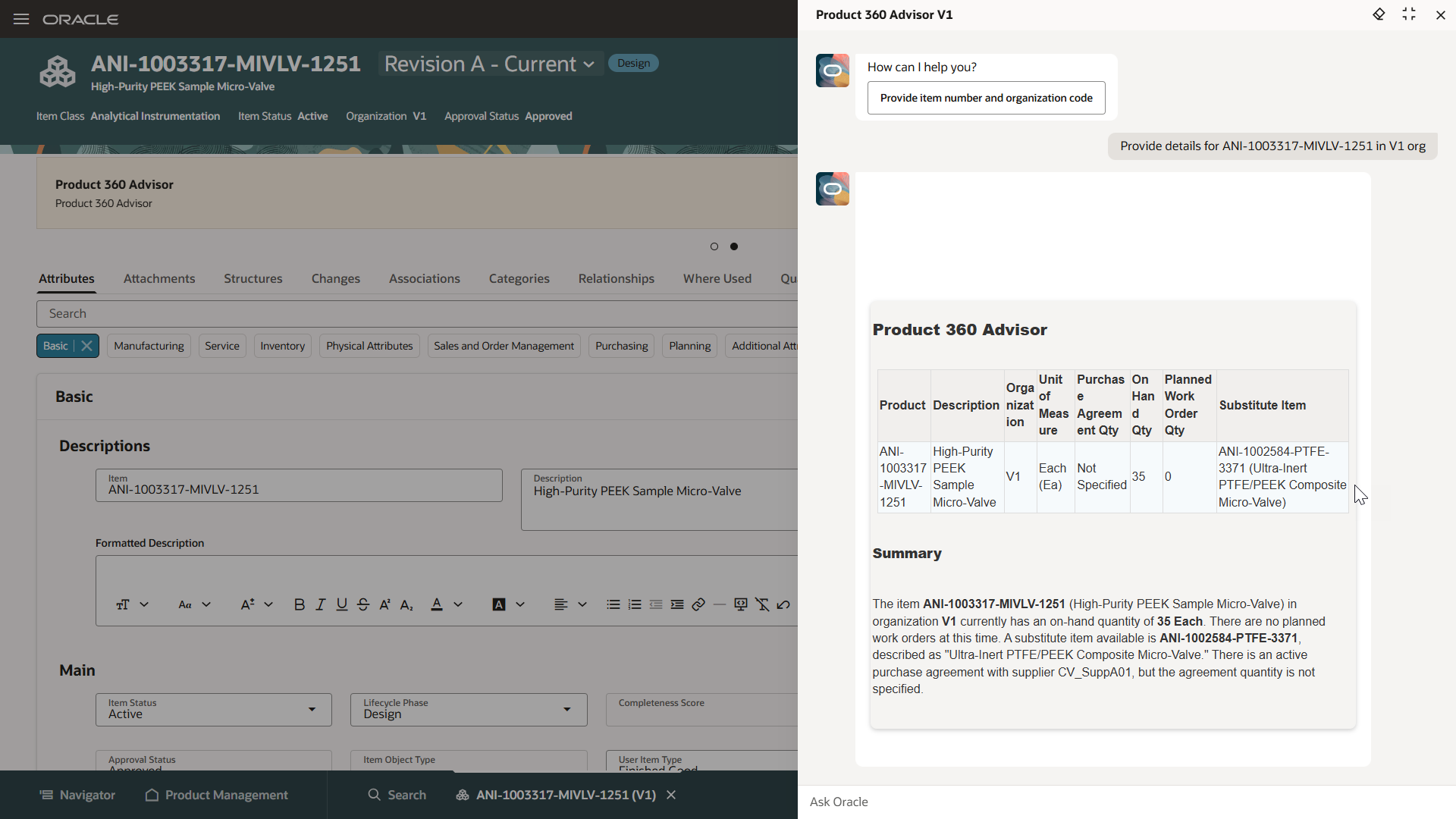
Uncover data-driven insights
Make faster, more informed decisions and reduce risk with a predictive and connected 360-degree view of product quality across product lifecycle and supply chain processes.
Integrated issue management
Drive greater efficiency with consistent controls of your quality processes. Capture, aggregate, and perform root-cause analysis on quality issues raised from sources including IoT, field service, and incoming and work-in-process inspections.
Streamline preventative actions
Rapidly respond to issues, prevent reoccurrence, and drive continuous improvement with closed-loop corrective action processes that are tightly integrated with your product development, enterprise change, document management, and inspection processes.
Centralized document management
Manage standard operating procedures and industry regulations with a single source of truth that integrates training, document management, and change control to drive operational consistency, compliance, process improvement, and audit readiness.
Product master data management (MDM)
Product commercialization
Efficiently commercialize new products and services by sharing the right product data required for your sales, marketing, supply chain, and ERP processes.
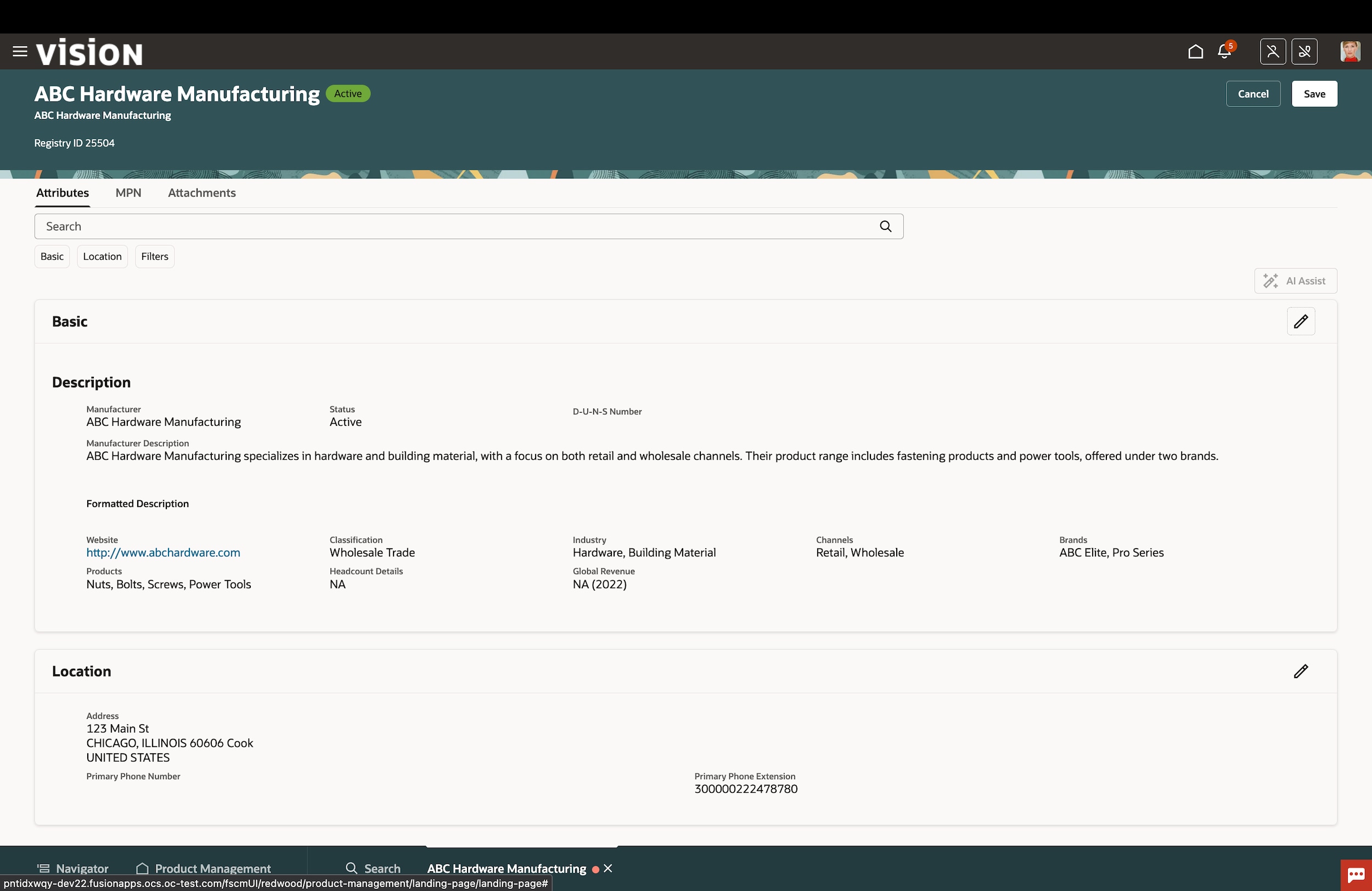
Item mastering
Keep your item master data clean and synchronized across applications, data pools, and partners with a best-practice product information management (PIM) process and flexible attribution, change control, and native-governance capabilities.
Omnichannel commerce
Ensure that complete and consistent product information is published across your sales channels while efficiently sharing trusted product data across back-end SCM, ERP, and order fulfillment systems.
Integrated collaboration for external users
Automate and deliver continuous business-rule validations and verification of data to allow global contract manufacturers and suppliers to receive accurate information and authenticate the data they provide—no matter where they are located.
ERP migration and consolidation
Gain a single platform to centralize and consolidate product data—without disrupting ERP and supply chain operations. Oracle Cloud PLM can help you unify data from numerous ERP systems as a result of organic growth or a merger or acquisition.
Configurator modeling
Model complex products
Leverage a dynamic design environment to build configurator models that deliver an intuitive user experience. Simplify the configuration of multioption, customizable products and services.
Simplify the sales process
Guide your customers to the right—or optimal—products. Simplify the configuration process by adding high-level questions and tying the answers directly to one or more option selections.
Optimize user experience
Build better user experiences by guiding your customers to valid product configurations. Use display condition rules and a variety of selection controls to dynamically control how your content is displayed in the user interface.
Apply templatized design
Quickly create the dynamic runtime user interface that best meets your needs by choosing from predefined user interface templates.
Simulate model behavior
Design, build, and test business logic, model behavior, and overall user experience prior to release. When releasing new versions, you can leverage comprehensive impact analysis and validation checks to ensure quality.
Learn how Oracle Cloud PLM helps your supply chain and development teams unify processes and effectively manage data.
Oracle Cloud PLM lets the data flow whenever and wherever a good idea happens to drive continuous innovation.
Learn how cloud-based PLM software can break down data silos to help support your business transformation.
PLM use cases
Connect your product value chain
Unifies processes—from capturing an idea through commercializing products and services—on a single data model for faster decision-making.
Your guide to modern Product Lifecycle Management (PLM) software (PDF)
Drive faster innovation to launch
Build a smarter innovation pipeline fueled by a steady stream of high-value ideas. Efficiently design, develop, and manage new product introductions and engineering change requirements.
Harmonize product data
Leverage a single enterprise product record that spans all applications to provide the velocity and resiliency needed to support today’s complex business transformations.
Read the analyst commentary: Weaving the Digital Thread: Oracle’s Enterprise Product Record
Reduce the cost of quality
Drive closed-loop quality management to make successful, high-speed decisions with real-time transparency and traceable information.
Learn how to reduce the cost of quality
Watch how companies are transforming manufacturing and quality across the supply chain
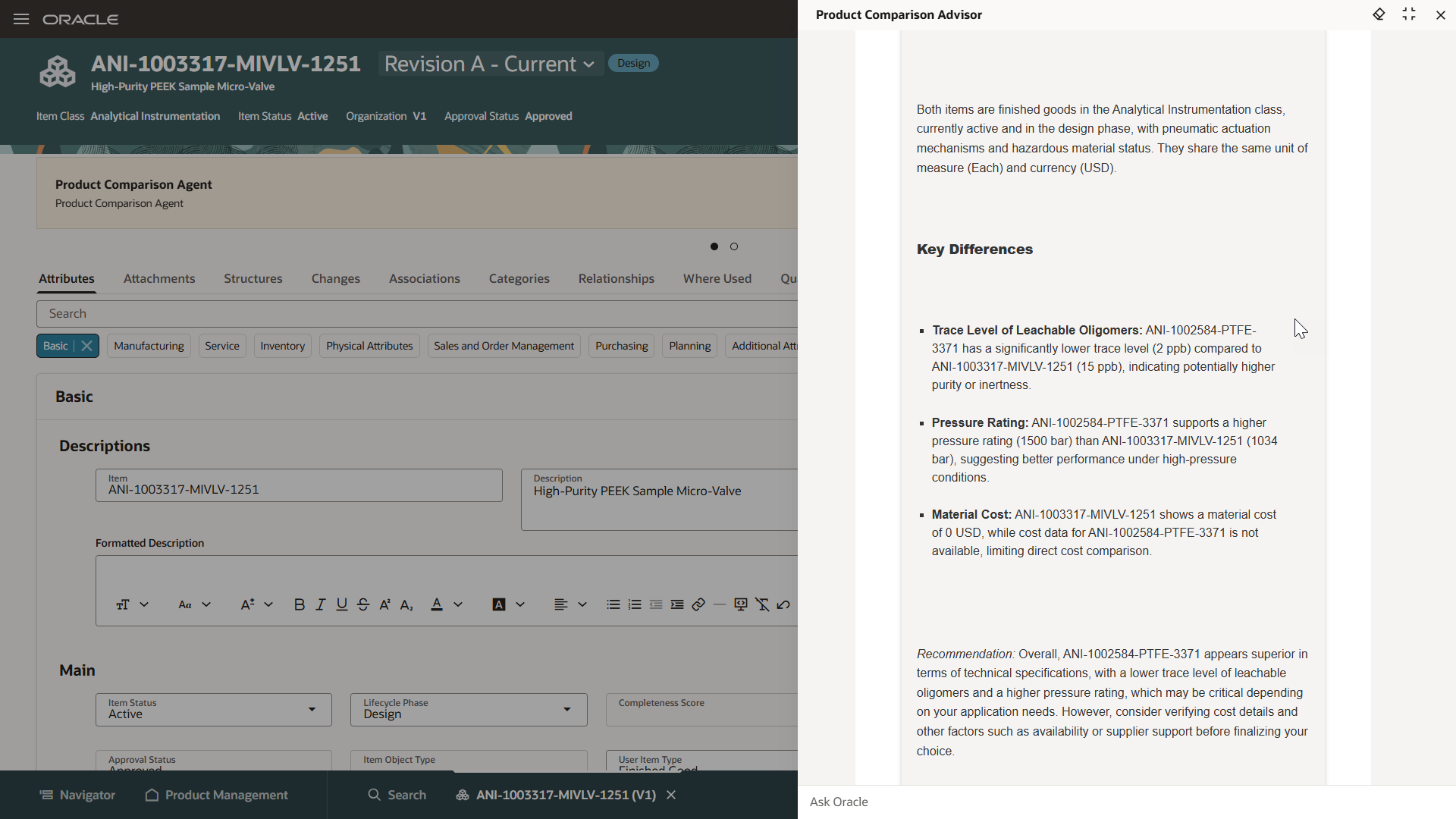
Leverage the latest technology
Get immediate value from built-in predictive analytics, artificial intelligence (AI), machine learning (MI), IoT, and digital assistance that work together to create digital twins and turn data into insights.
Product tour—Product Lifecycle Management (PLM)
Enable a Digital Thread for Supply Chain Transformation
Top 5 Must-See Oracle Product Lifecycle Management 26A Enhancements
Lucia Ligamari, Manager, Product Lifecycle Management and Procurement Product Marketing
Something big just landed for Oracle Fusion Cloud Product Lifecycle Management 26A, and it’s set to change the way you manage products and data. Check out our countdown of the major updates that are driving smarter workflows, better visibility, and a next-level user experience. The details might surprise you.
Read the complete postResources

Learn what's new in the latest PLM release
Review Cloud update readiness material to learn what's new in your Oracle Cloud PLM service and plan for quarterly updates.

Access our documentation library
Oracle Help Center provides detailed information about our products and services with targeted solutions, getting started guides, and content for advanced use cases.

Join a community of your peers
Cloud Customer Connect is Oracle's premier online cloud community. With more than 200,000 members, it's designed to promote peer-to-peer collaboration and sharing of best practices, product updates, and feedback.

Develop your Cloud PLM skills
Oracle University provides you with free training and certification to ensure your organization’s success, delivered in your choice of formats.
Page
Oracle Product Lifecycle Management Cloud Demo Showcase
Related products
Get started with Oracle PLM
Cloud readiness
Learn about the latest innovations.
Take a tour
Explore PLM on your own.
Contact SCM sales
Talk to a member of our team about PLM.